recomLine Borrelia
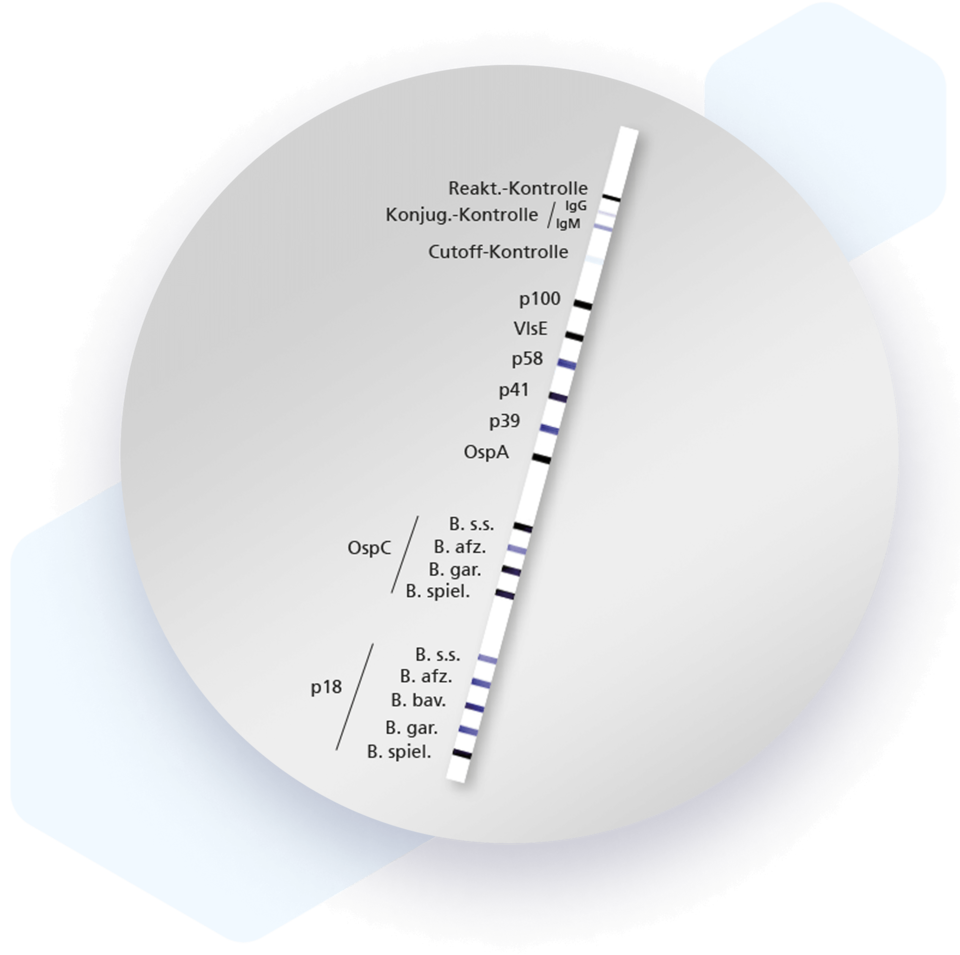
Mikrogen recomLine Borrelia Tests are serological, qualitative in vitro tests with recombinantly produced, highly specific and immunodominant Borrelia antigens. All specific antibodies against all known Lyme disease-causing Borrelia species (B. burgdorferi sensu stricto, B. garinii, B. afzelii, B. spielmanii and B. bavariensis) can be detected on one test strip.
The recomLine Borrelia tests are used to confirm the results of screening enzyme immunoassays.
Products
Downloads
Advantages
- Optimal imaging without cross-reacting Borrelia proteins
- Identification of early and late infection status through different, typical antigen band patterns
- Standardized serum CSF analysis possible, for detection of antibody index in suspected cases of Lyme neuroborreliosis
- Separate detection of IgG and IgM antibodies
- Simple and clear interpretation due to easy-to-read banding
- Compliant with MiQ Lyme Borreliosis1 and DIN 58969-442
- Partial and full automation, software-based evaluation (recomScan) and integration with laboratory information system possible
- Highest sensitivity and specificity by using recombinant, immunodominant antigens:
| Antigen | Tribe |
| p100 | B. afzelii |
| VIsE | different Borrelia genospecies |
| p58 | B. garinii |
| p41 | B. burgdorferi sensu stricto |
| p39 | B. afzelii |
| OspA | B. afzelii |
| OspC | B. burgdorferi sensu stricto, B. afzelii, B. garinii, B. spielmanii |
| p18 | B. burgdorferi sensu stricto, B. afzelii, B. garinii, B. spielmanii, B. bavariensis |
Safe and reliable
Validity check by integrated, strip-specific cutoff and antibody class controls
Flexible and compatible
Combination of all microgen line immunoassays possible - uniform processing and interchangeable reagents
High Standard
CE mark: The recomLine Borrelia tests meet the high standard of the EC Directive 98/79/EC for in vitro diagnostic medical devices.