recomLine HantaPlus
Line immunoassays for the detection of IgG and IgM antibodies
against hantavirus and sandfly fever virus in human serum and plasma.
against hantavirus and sandfly fever virus in human serum and plasma.
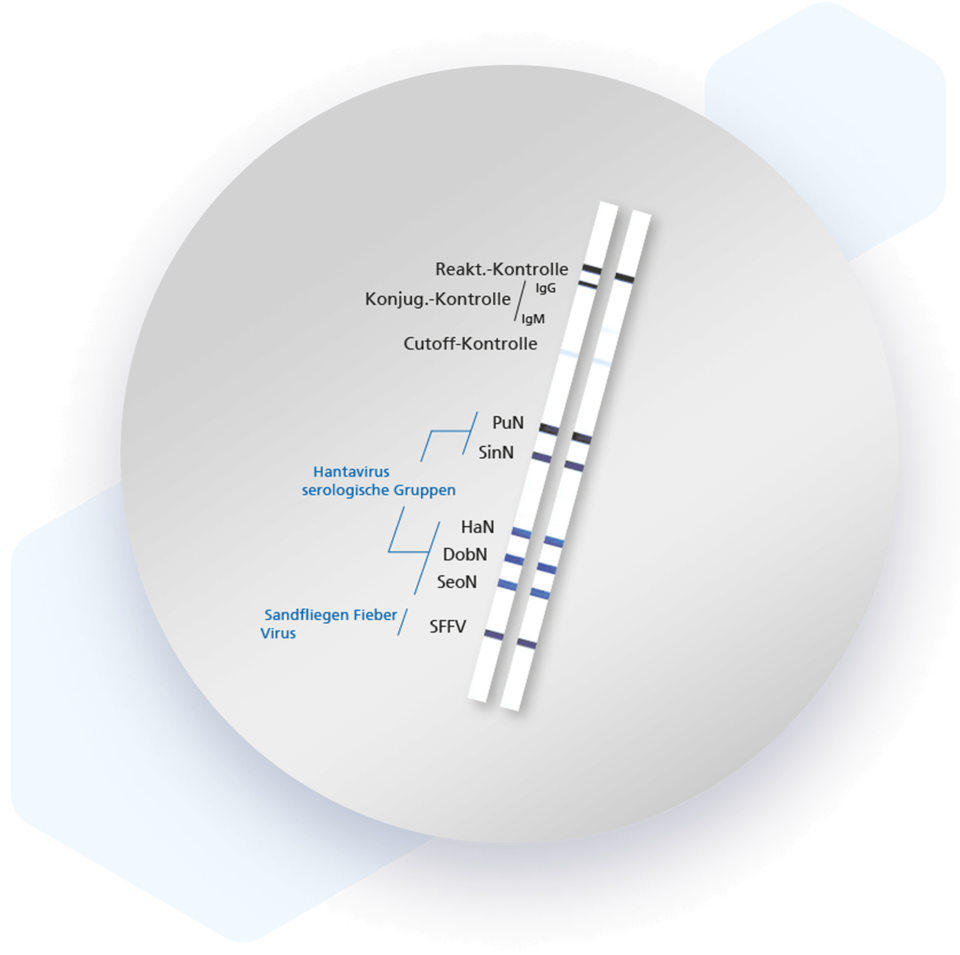
Mikrogen recomLine HantaPlus tests are serological, qualitative in vitro line immunoassays based on recombinantly produced antigens for the detection of IgG and IgM antibodies against Hantavirus (serotypes Puumala, Sin Nombre, Hantaan, Dobrava and Seoul) and Sandfly Fever Virus (serotypes Tuscany and Sicily).
Products
Downloads
Advantages
- Early stage diagnosis through cost-effective, serological IgM detection
- Detection of different hantavirus serotypes, which are grouped into two serological groups due to their homology
- Serotyping by IgG results in combination with travel history possible
- More moderate courses with less/without use of intensive care or dialysis (costs)
- Developed in collaboration with the German Reference Laboratory for Hantaviruses
- Simple and clear interpretation due to easy to read banding
- Partial and full automation, software-based evaluation (recomScan) and integration with laboratory information system possible
- Highest sensitivity and specificity through the use of recombinant antigens:
| Band | Description |
| PuN | Serotype-specific N-terminus of the Puumala virus nucleocapsid antigen |
| SinN | Serotype-specific N-terminus of the Sin Nombre virus nucleocapsid antigen |
| HaN | Serotype-specific N-terminus of the Hantaan virus nucleocapsid antigen |
| DobN | Serotype-specific N-terminus of the Dobrava virus nucleocapsid antigen |
| SeoN | Serotype-specific N-terminus of the Seoul virus nucleocapsid antigen |
| SFFV | Complete sandfly fever virus nucleocapsid antigen serotype Tuscany and Sicily |
Safe and reliable
Validity check by integrated, strip-specific cutoff and antibody class controls
Flexible and compatible
Combination of all microgen line immunoassays possible - uniform processing and interchangeable reagents
High Standard
CE mark: The recomLine HantaPlus tests meets the high standard of the EC Directive 98/79/EC for in vitro diagnostic medical devices.