recomLine HSV-1 & HSV-2 IgG
against HSV-1 and HSV-2 in human serum or plasma
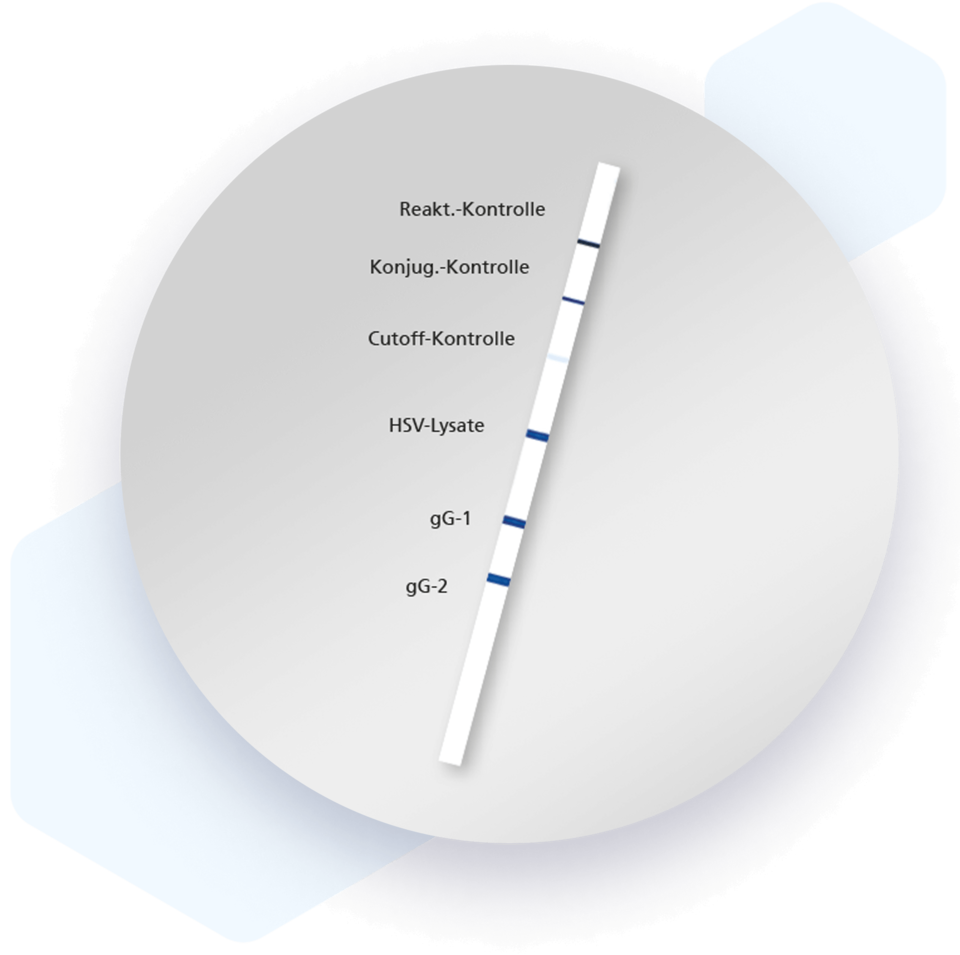
Mikrogen recomLine HSV-1 & HSV-2 IgG Test is a serological, qualitative in vitro line immunoassay based on purified, subtype-specific recombinant gG1 (HSV-1) and gG2 (HSV-2) antigens, ensuring accurate differentiation of HSV-1 and HSV-2 infections.
Most serologic methods for determining HSV status use viral lysate as antigens and cannot distinguish between HSV-1 and HSV-2 infections due to cross-reactivity. Since most adults have already had an HSV-1 infection (seroprevalence is approximately 83% in Germany), serological status for HSV-2 cannot be reliably diagnosed using viral lysates.
Products
| Product | Size | Article no. | |
|---|---|---|---|
|
Reagents for 20 determinations
|
Article no.: 5372 | Request |
Downloads
Advantages
-
Highest sensitivity and specificity due to the use of recombinant antigens: gG1 (HSV-1) and gG2 (HSV-2)
-
Application as serological confirmation or supplement for PCR and/or cell culture findings, after positive screening with HSV ELISA
-
Application for determination of subtype-specific HSV serostatus of risk groups:
-
Herpes genitalis positive patients and their partners.
-
Patients with an increased risk for sexually transmitted diseases
-
Immunosuppressed patients (e.g. HIV seropositive, transplant patients)
-
Recurrent genital and anal infections
-
Pregnancy (risk assessment of primary HSV infection or risk of herpes neonatorum)
-
- No cross reactions
-
Easy and clear interpretation due to easy-to-read banding
-
Partial and full automation, software-based evaluation (recomScan) and integration with laboratory information system possible
Safe and reliable
Validity check by integrated, strip-specific cutoff and antibody class controls
Flexible and compatible
Combination of all Microgen line immunoassays possible - uniform processing and interchangeable reagents
High Standard
CE mark: The recomLine HSV-1 & HSV-2 test meets the high standard of the EC Directive 98/79/EC for in vitro diagnostic medical devices.