recomLine Tropical Fever
against Dengue (DENV), Chikungunya (CHIKV) and Zika virus (ZIKV) infections in human serum or plasma
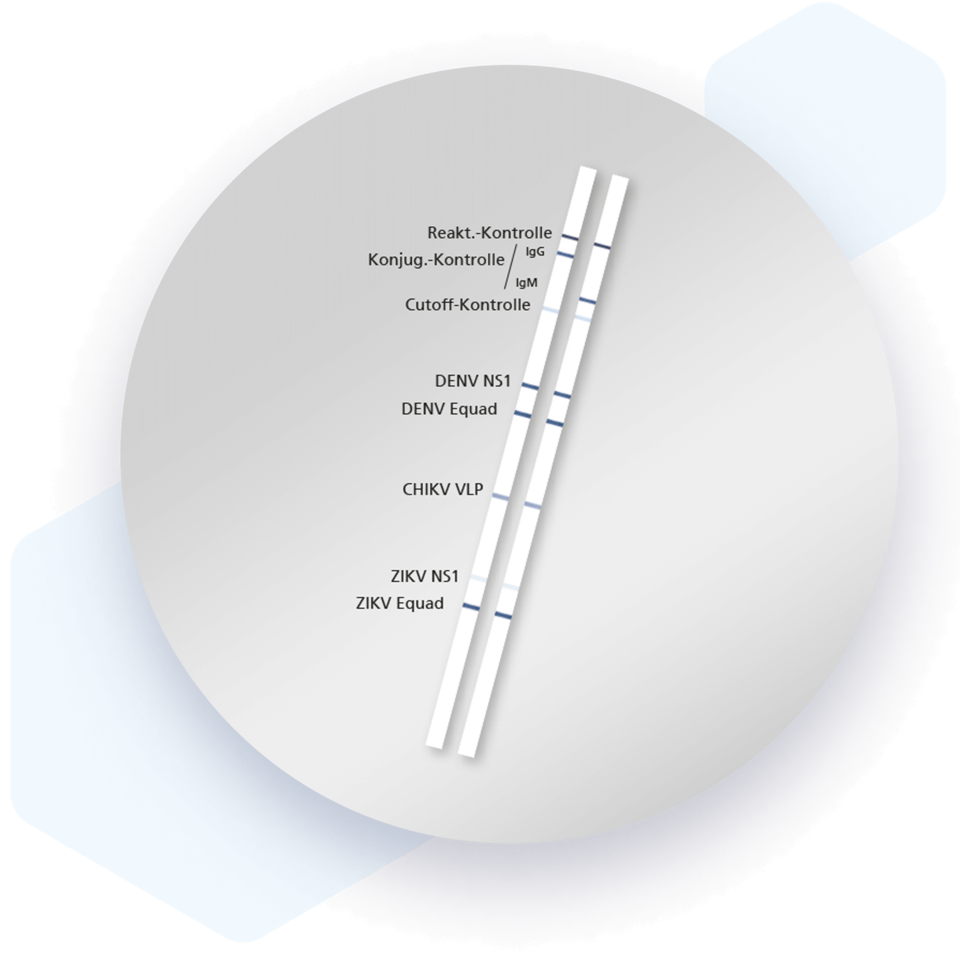
Mikrogen recomLine Tropical Fever tests are serological, qualitative line immunoassays that combine the diagnostic markers Equad proteins and non-structural proteins (NS1), thereby enabling the identification of specific antibodies against the individual antigens of Dengue, Chikungunya and Zika viruses. Virus-like particles (VLP) are used to detect CHIKV and NS1 and Equad proteins are used for DENV and ZIKV. The unique setup allows differentiation of DENV and ZIKV using a 2-step interpretation scheme that includes NS1 antigen reactions and, in their absence, reactivities against Equad proteins.
Products
Advantages
- New antigens: higher sensitivity due to CHIKV VLP, DENV and ZIKV Equad as well as improved flavivirus differentiation
- Simultaneous detection and differentiation of DENV, CHIKV and ZIKV infections in one step
- Parallel detection of IgG and IgM enables monitoring of seroconversions and supports diagnosis of both primary and secondary flavivirus infections
- Additional diagnostic support to reliably exclude ZIKV infections in pregnant women
- For human serum/plasma from approximately 7 days after symptom onset and for follow-up samples after 1-2 weeks
- Easy and clear interpretation due to two-band criterion
- Partial and full automation, software-based evaluation (recomScan) and integration with laboratory information system possible
- High sensitivity and specificity due to the use of recombinant antigens:
| Antigen bands | Description |
| DENV NS1 | Dengue non-structural protein 1 (1st stage differentiation). |
| DENV Equad | Variant d. dengue envelope protein with targeted mutations to increase specificity (2nd stage differentiation). |
| CHIKV VLP | Chikungunya virus-like particles |
| ZIKV NS1 | Zika non-structural protein 1 (1st stage differentiation). |
| ZIKV Equad | Variant d. Zika envelope protein with targeted mutations to increase specificity (2nd stage differentiation). |
Safe and reliable
Validity check by integrated, strip-specific cutoff and antibody class controls
Flexible and compatible
Combination of all Mikrogen line immunoassays possible - uniform processing and interchangeable reagents
High Standard
CE mark: CE marked and patent pending